How do hand sanitizers kill germs?
Hand sanitizers have become a staple in our daily routines, especially with the heightened awareness of hygiene due to global health challenges. Understanding how these products work to kill germs is crucial for maximizing their effectiveness and ensuring our safety. This article will delve into the science behind hand sanitizers, the key ingredients that contribute to their germ-killing abilities, and the mechanisms by which they act. We will also provide best practices for using hand sanitizers safely and effectively.
Understanding the Science Behind Hand Sanitizers’ Effectiveness
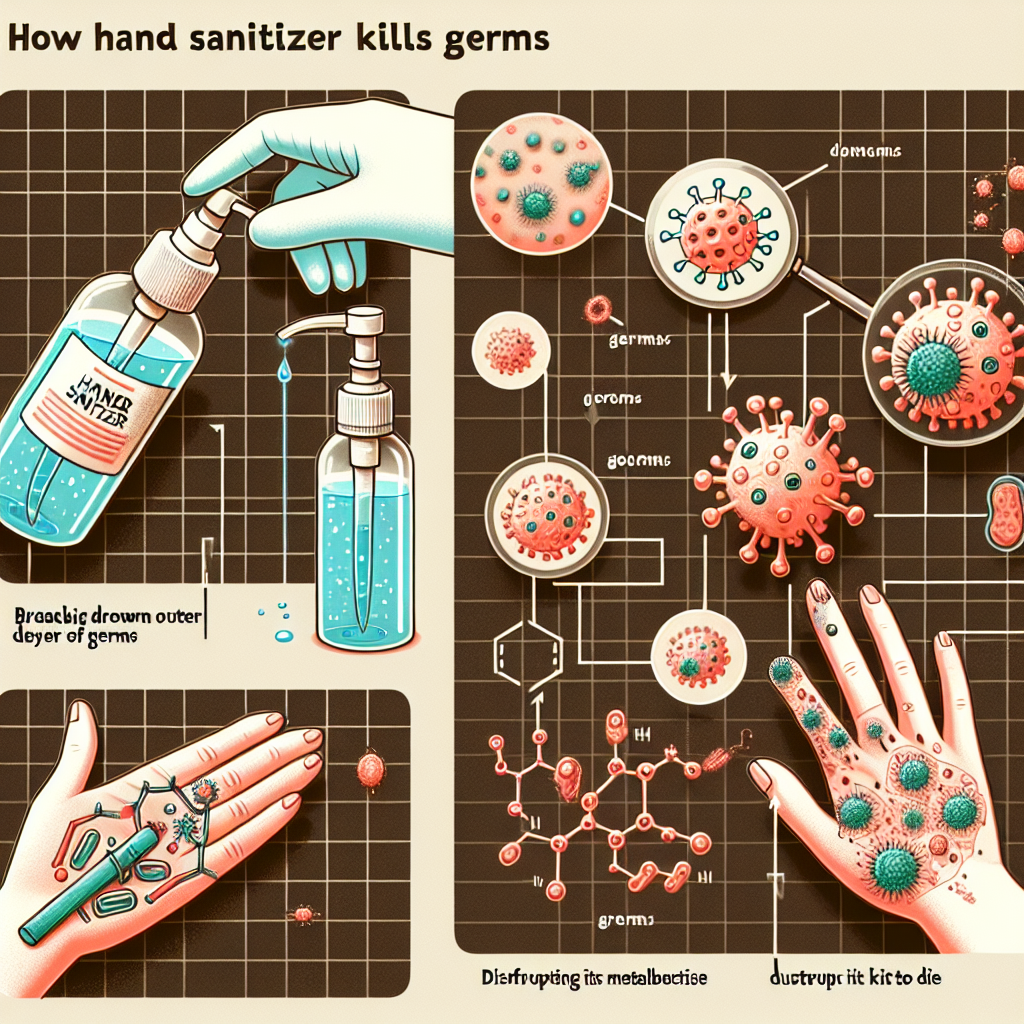
Hand sanitizers are designed to eliminate a wide range of pathogens, including bacteria and viruses, from the skin’s surface. Their effectiveness largely hinges on their chemical composition, which can disrupt the cellular structure of germs. The Centers for Disease Control and Prevention (CDC) recommends sanitizers with at least 60% alcohol concentration for effective germ killing. This high alcohol content is crucial because it ensures that enough active ingredient is present to penetrate and disrupt microbial cell walls.
The science behind hand sanitizers involves understanding the types of germs that commonly inhabit our hands. These include transient flora, which we pick up from surfaces, and resident flora, which are the microorganisms already present on our skin. While washing hands with soap and water is effective in removing dirt and germs, hand sanitizers provide a convenient alternative when soap is not available. Their rapid action allows for quick disinfection, making them particularly valuable in settings like healthcare facilities and during public gatherings.
However, it is important to note that not all hand sanitizers are created equal. The effectiveness of a hand sanitizer can vary based on its formulation and the types of microorganisms it is intended to combat. For instance, hand sanitizers may not be as effective against certain types of germs, such as Clostridium difficile spores, which require more robust cleaning methods. Thus, understanding the science behind hand sanitizers is essential for informed usage and maximizing their benefits. For further reading, check out the CDC’s guidelines on hand hygiene.
Key Ingredients in Hand Sanitizers and Their Functions
The primary active ingredient in most hand sanitizers is alcohol, which can be in the form of ethanol or isopropyl alcohol. This ingredient is responsible for the rapid and effective action that sanitizers are known for. Alcohol works by denaturing proteins and dissolving lipids, which effectively disrupts the cellular membranes of bacteria and viruses. In addition to alcohol, many formulations also contain emollients, such as glycerin or aloe vera, which help to mitigate the drying effect of alcohol on the skin.
Other ingredients commonly found in hand sanitizers include hydrogen peroxide, which serves as an additional antimicrobial agent. While its concentration in hand sanitizers is typically low, it can help enhance the overall effectiveness of the product. Additionally, some manufacturers include thickening agents to improve the texture and usability of the sanitizer, ensuring it adheres well to the skin during application.
Fragrances and colorants are often added to provide a pleasant sensory experience but serve no functional purpose in germ-killing. However, they can enhance user compliance, encouraging more frequent use of hand sanitizers. It’s essential for consumers to be aware of these ingredients and their roles to make informed choices about the products they use. For an extensive list of common ingredients in hand sanitizers, you can explore FDA resources.
The Mechanism of Action: How Sanitizers Destroy Germs
The effectiveness of hand sanitizers lies in their ability to penetrate the outer membrane of microbial cells. Alcohol-based sanitizers work primarily through a process called protein denaturation, where the high concentration of alcohol causes proteins within the germ to unfold and lose their functional structure. This interruption in protein function renders the germ inactive and unable to replicate.
Once the alcohol penetrates the germ’s cell wall, it also disrupts the lipid bilayer, which is critical for maintaining the integrity of the cell. This dual action—denaturing proteins and disrupting lipids—ensures that both bacteria and viruses are effectively neutralized. However, it is important to apply the sanitizer generously and rub it thoroughly over all surfaces of the hands for at least 20 seconds to ensure maximal effectiveness.
Additionally, some hand sanitizers employ a mechanical action known as "liquid action," where the gel or liquid formula physically removes germs from the skin. While this is not as effective as the chemical action of alcohol, it can still contribute to the overall cleansing process. Nevertheless, understanding that the primary mode of action is chemical disruption is essential for evaluating hand sanitizer efficacy. For more insights into microbial mechanisms, consider reading about antimicrobial resistance.
Best Practices for Using Hand Sanitizers Safely and Effectively
To maximize the effectiveness of hand sanitizers, ensure that you apply a sufficient amount to cover all surfaces of your hands. The CDC recommends using enough product to cover your hands entirely, typically about the size of a quarter. After application, rub your hands together until they feel dry, which should take around 20 seconds. This duration allows the alcohol to have sufficient contact time with the germ to ensure effective killing.
It is also crucial to understand when hand sanitizers are appropriate to use. While they are effective in most situations, they are not a substitute for handwashing with soap and water, especially when hands are visibly dirty or greasy. In such cases, hand sanitizers may not be able to penetrate and remove debris effectively. Therefore, maintaining a balance between using sanitizers and traditional handwashing is essential for optimal hygiene.
Lastly, store hand sanitizers correctly to maintain their effectiveness. Keep them in a cool, dry place, away from direct sunlight, as heat and light can evaporate the alcohol and reduce its efficacy. Additionally, be cautious of the alcohol content in hand sanitizers, as they are flammable. Following these best practices can significantly enhance your hand hygiene routine and help prevent the spread of germs. For more detailed guidelines on hand hygiene practices, refer to the World Health Organization’s (WHO) hand hygiene resources.
In summary, hand sanitizers are a critical tool in our fight against germs, particularly in settings where soap and water may not be readily available. By understanding the science behind their effectiveness, the key ingredients they contain, and their mechanisms of action, we can make informed decisions about when and how to use these products. As we navigate an increasingly health-conscious world, adhering to best practices for hand sanitizer use will empower us to maintain our personal hygiene and prevent the spread of infectious diseases. Stay informed, stay safe, and prioritize your health.
Top hand sanitizers for virus protectionSeasonal flu vaccine effectivenessLatest research on virus transmissionRelevant LinkRelevant LinkRelevant Link